Electric charge
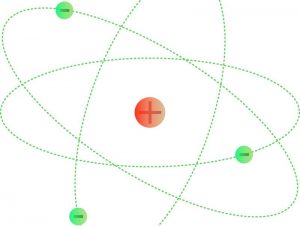
In physics, electrical charge is a property of matter that is responsible for producing electrostatic interactions. Matter is composed of atoms which in turn are composed of electrons with a negative electrical charge and protons with a positive electrical charge. Protons are found in the atom nucleus and electrons in the cortex revolving around the nucleus.
- Unit: coulomb
- Symbol: "e"
- Formula: e = 1,602 x 10-19 coulombs
What is the electric charge?
The electric charge is a unit of measurement in the International System of Units that determines a particle's ability to exchange photons. It can also be defined as a physical property of some subatomic particles manifested through repulsion forces and attraction between them by the interaction of electromagnetic fields. Electric charges can be positive or negative.
Properties of the electric charge
Electric charge have the following properties:
- It is a quantized magnitude, which means that any type of body is always a multiple of the value of “e”.
- There are three types of charges, the positive (when there are fewer electrons than protons), the negative (when there are more electrons than protons) and the neutral (when there are equal numbers of protons and electrons). Equal charges are rejected, and different charges are attracted.
- The attraction force or repulsion between charges varies with the inverse square of their separation distance.
- Electric charges are neither created nor destroyed because their value is constant.
- The charges that allow the movement on the surface of certain bodies are called conductors and those that do not, are known as insulators.
Symbol
The electric charge symbol is “e” and its formula is: e = 1.602.10-19 coulombs.
Units
The unit of the electric charge is the coulomb, symbolized by the letter C according to the International System of Units.
C is equal to the amount of charge it exerts on another charge equal to the distance of one meter.
Types of electric charge
There are several types of electrical charges: Positive, negative, inductive, resistive, capacitors and combined.
- Positive electrical charges are those that have a higher charge of protons than electrons or that have positive polarity in their totality.
- Negative electrical charges are those with a higher charge of electrons than protons, or which have a negative polarity in their totality.
- Resistive electrical charges are those that can resist electricity flow dissipating a certain amount of this energy in the form of heat.
- Inductive electric charges are those that have a type of conductive material that when in contact with the electric current generates a magnetic field around it.
- Capacitor electric charges have a capacitor that allows them to store energy and a non-conductive insulator on both conductive surfaces. This produces that at the moment that the capacitor makes contact with the electrical energy, electrons accumulate around the plate attached to the terminal, where the electrical current is applied.
- Combined electric charges are those that can combine capacitors, inductors and resistors to perform specific functions.
Electrical charge of the electron
The electrical charge of the electron is negative. This is represented by the symbol “e.
Of the proton
The electrical charge of the proton is positive. It is represented by the following formula 1 (1,602 x 10-19 coulombs).
Of the atom
The electrical charge of the atom can be positive, negative or neutral. It all depends on how many protons or electrons it has. If the number of protons is more than the electrons, the atom will have a positive charge; if the number of electrons is greater than the protons, the charge of the atom will be negative; but if the quantity of electrons and protons is the same, the charge will be neutral.
The neutron is the nucleus of an atom and does not possess an electric charge. It is made up of three charged particles called quarks, whose charges sum zero.
Quantization
It is not manifested in any quantity, but in multiples of wholes of a unit based on the electron electrical charge.
In this sense, electric charge quantization occurs when whole quantities of electric charge, multiples of elemental charge and electric charge are transferred in an electric process.
The quantization of the electric charge is calculated with the formula Q = N * e.
Examples
Here are some examples of electrical charging:
- If you rub a balloon against your hair and then approach it to the wall, the balloon will remain attached to it, because of the electric force of attraction between the wall and the balloon.
- The clouds’ friction with the air causes the clouds to be charged with electricity and then, electrical discharges into the air.
- After combing it is possible to lift small pieces of paper with the comb. This happens because the comb is charged by hair electrons that are negative and then, they attract the paper charges and because of that, they adhere to the comb.
- By rubbing a plastic spoon with a flannel, the spoon acquires a negative charge. If we then approach the spoon to a jet of water, the water drop will be diverted because the negative charge of the water and the spoon are rejected.
How to cite this article?
Briceño V., Gabriela. (2019). Electric charge. Recovered on 4 January, 2025, de Euston96: https://www.euston96.com/en/electric-charge/