Ozone layer
Most of the Earth's atmospheric ozone is concentrated in one of the stratospheric layers, approximately 15 to 30 km above Earth's surface. Ozone is a molecule containing three oxygen atoms that can only exist in specific circumstances where temperature and pressure are adequate. The ozone layer acts as a radiation barrier and allows ultraviolet light to pass through at the same time.
What is the ozone layer?
The ozone layer is the layer located in the stratosphere that contains, as its name indicates, ozone, which is responsible for playing a protective barrier role against solar radiation and also allows the passage of ultraviolet light, necessary for life.
What is the ozone layer for?
The main function of the ozone layer is to absorb ultraviolet radiation from the Sun, thus protecting the Earth against its harmful effects. Without the existence of the ozone layer in the atmosphere, it would be very difficult for any living being to survive on the surface.
Where it is found?
The layer of the atmosphere that extends between 15 and 60 kilometers is called the stratosphere. This is the layer in which we can locate the ozone layer, which is found mainly in the lower portion of the stratosphere about 20 to 30 kilometers above the earth’s surface, although this measure of thickness can vary seasonally and geographically.
Who discovered her?
Initially, it was thought that ozone had been identified in 1839 by Christian Schonbein, a Swiss chemist who studied and looked for electrical discharges. When he found it, he gave it the name ozone, a word derived from the Greek term “to smell”, because it had a strong smell that was similar to the smell of electric wires when ignited in flames. However, Schonbein failed to adequately describe the molecular structure of ozone, which the scientist Jean-Luis Soret did in the 1860s. Despite his failure, Schonbein kept up his research and was one of the first to indicate that higher ground level ozone concentrations could affect people’s health.
Characteristics
Its main characteristics are:
- Its color is blue in liquid form and red in solid form.
- It has a certain odor.
- It has more instability in the air than water.
- It occurs naturally in the atmosphere.
- It retains 90% of the sun’s ultraviolet rays.
- It is formed by ozone molecules.
Function
The ozone layer acts as a filter against ultraviolet radiation, which has a shorter wavelength and is highly dangerous, therefore protecting both animal and plant life on earth from its potentially harmful effects.
Pollution of the ozone layer
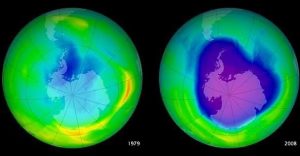
In the course of the 20th century, the ozone layer has suffered serious alterations, mainly its weakening. This problem has been called the ozone hole. Today, there is widespread concern that the ozone layer is deteriorating due to the release of pollution from chemicals, chlorine and bromine.
Destruction of the ozone layer
Hole
The hole in the layer is an area where there are abnormal reductions in the amount of ozone. This phenomenon occurs most rapidly during spring and in the pole regions. It is produced by the increase of chlorine and bromine in the layer known as the stratosphere by the emanation of chemical compounds. The Antarctic “ozone hole” was revealed by scientists Joe Farman, Brian G. Gardiner and Jon Shanklin, and they discovered that there was a 50% reduction.
Causes
Chlorofluorocarbons are chemical substances found mainly in aerosols used by industries over the last 50 years, they are the main reasons for ozone layer degradation. When these substances reach the upper atmosphere, they are exposed to ultraviolet rays, which causes them to break down into substances that include chlorine. Chlorine reacts with oxygen atoms in ozone and tears the ozone molecule. The ozone layer over Antarctica has been particularly affected by pollution since the mid-1980s. Low temperatures in this region accelerate the conversion of CFCs into chlorine, which rapidly and massively destroys the ozone layer. The main countries that have damaged the layer have been the United States and Europe because of the number of industries they possess.
Consequences
This layer damage allows large amounts of ultraviolet rays to reach the Earth, and this can have devastating consequences for humans, such as skin cancer and cataracts in humans. This radiation inhibits the reproductive cycle of phytoplankton and biologists fear that reductions in phytoplankton populations will decrease the populations of other animals. Researchers have also documented changes in the reproductive rates of young fish, shrimp and crabs, as well as frogs and salamanders exposed to excess ultraviolet B radiation. This damage to the layer allows large amounts of ultraviolet rays to reach the Earth, and this can have devastating consequences for humans, such as skin cancer and cataracts in humans.
How to take care of the ozone layer
Some of the things we can do to help maintain and protect the ozone layer are the following:
- Use air conditioning and refrigeration equipment that does not use HCFC as a refrigerant.
- Buy aerosol products that do not use HCFCs or CFCs.
- Perform regular maintenance of air conditioning and refrigeration equipment to prevent and minimize refrigerant leaks.
- Recover or recycle refrigerant when a general overhaul of equipment is conducted.
- When vehicle air conditioners need to be repaired, they should be properly recycled rather than sent into the atmosphere.
- Have greater control in industries that release ozone depleting particles.
- Avoid using aerosols, lacquers, and cleaning products that damage the ozone layer.
Benefits
Ozone helps protect life on Earth by absorbing ultraviolet radiation from the sun, particularly UVB radiation that can cause skin cancer, cause cataracts in the eyes, damage crops and destroy some types of marine life.
How to cite this article?
Briceño V., Gabriela. (2019). Ozone layer. Recovered on 4 January, 2025, de Euston96: https://www.euston96.com/en/ozone-layer/